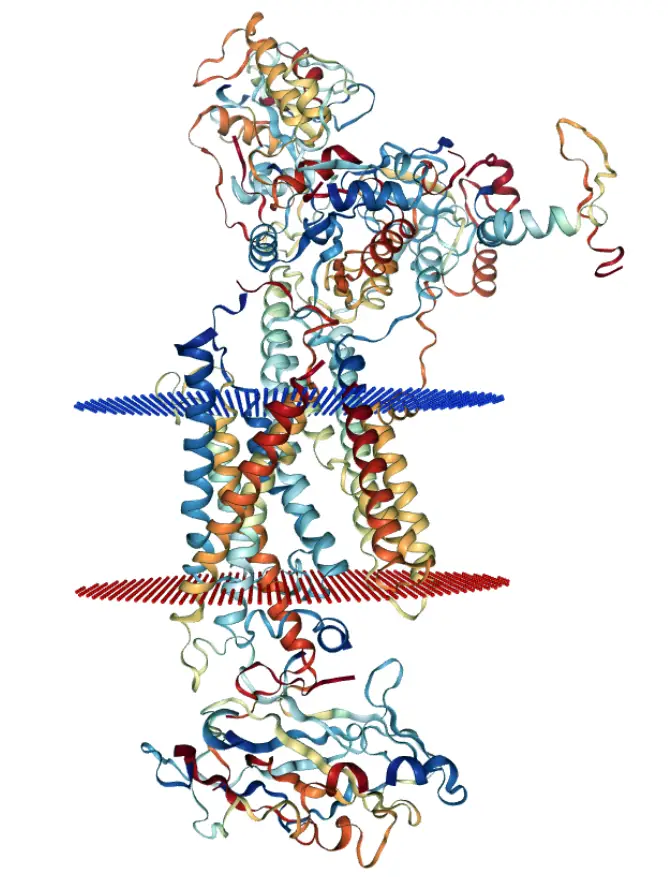
Bonds of Life: “Structural Plasticity” – Lipid mediated transmission and conformational toggle switch (an extract from the SB preprint)
Dyanmics of inter/intra molecular interactions were mapped and characterised using MD simulations of HKA at E2 like state. Among the transient network of salt-bridges, in particular the Salt-Bridges analysis of charged intrinsically disordered N-terminus of alpha subunit showed lipid-mediated salt-bridges. These salt-bridges of N-terminus, protein (e.g. GLU-pair) and lipids could regulate a toggle-switch for a communication/transmission pathway from cytoplasmic surface to TM catalytic unit and a function that is analogous to thermosensor kinase, DesK of bacteria Bacillus subtillis, promoting conformational changes.
“The thermosensor DesK is a multipass transmembrane histidine-kinase that allows the bacterium Bacillus subtilis to adjust the levels of unsaturated fatty acids required to optimize membrane lipid fluidity. The cytoplasmic catalytic domain of DesK behaves like a kinase at low temperature and like a phosphatase at high temperature. Temperature sensing involves a built-in instability caused by a group of hydrophilic residues located near the N terminus of the first transmembrane (TM) segment. These residues are buried in the lipid phase at low temperature and partially “buoy” to the aqueous phase at higher temperature with the thinning of the membrane, promoting the required conformational change. Nevertheless, the core question remains poorly understood: How is the information sensed by the transmembrane region converted into a rearrangement in the cytoplasmic catalytic domain to control DesK activity? Here, we identify a “linker region” (KSRKERERLEEK) that connects the TM sensor domain with the cytoplasmic catalytic domain involved in signal transmission. The linker adopts two conformational states in response to temperature-dependent membrane thickness changes: (i) random coiled and bound to the phospholipid head groups at the water-membrane interface, promoting the phosphatase state or (ii) unbound and forming a continuous helix spanning a region from the membrane to the cytoplasm, promoting the kinase state. Our results uphold the view that the linker is endowed with a helix/random coil conformational duality that enables it to behave like a transmission switch, with helix disruption decreasing the kinase/phosphatase activity ratio, as required to modulate the DesK output response.” \cite{inda2014lipid}, \cite{Albanesi2009structural}